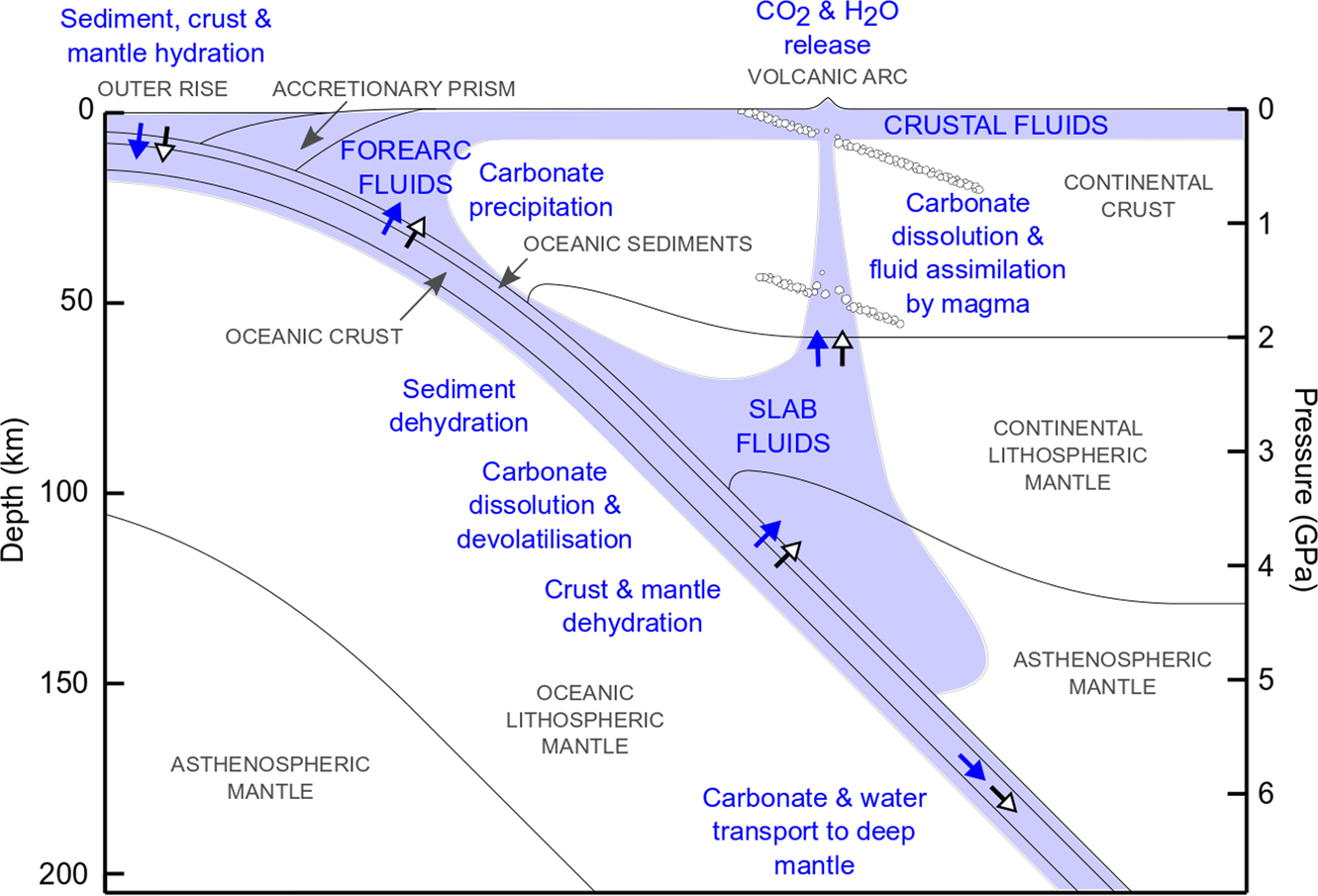
Self-accelerating volumetric dolomite-for-calcite replacement: A possible mechanism for high-temperature dolomitization? | SpringerLink
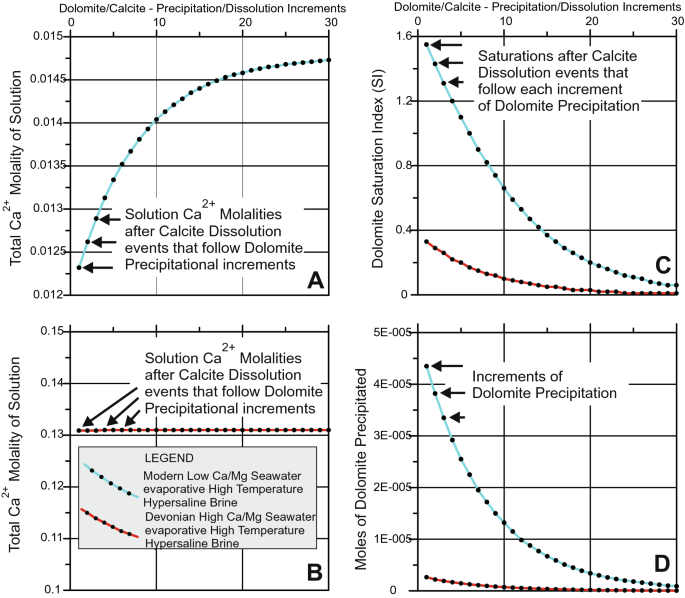
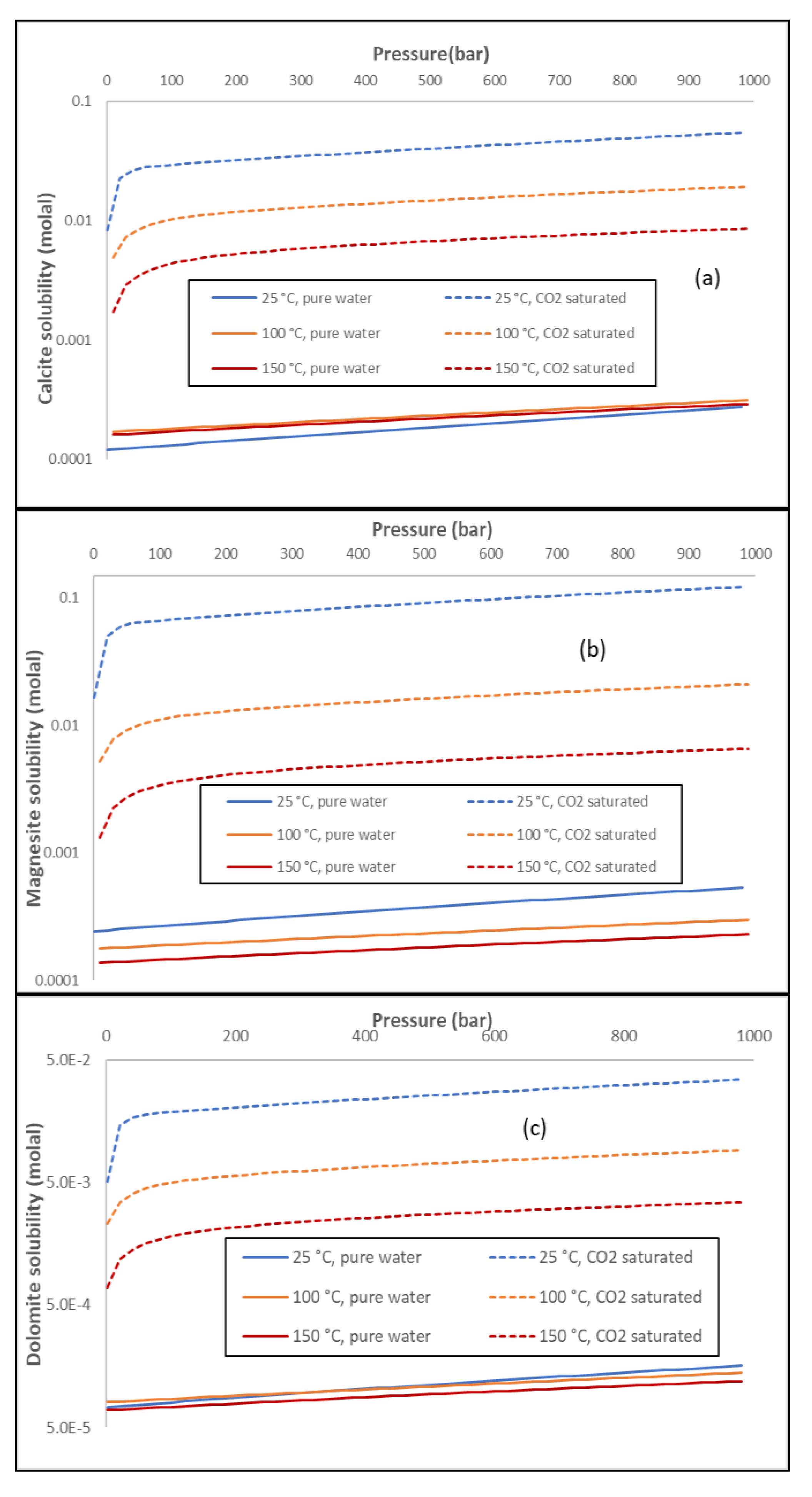
Energies | Free Full-Text | Thermodynamic Modeling of CO2-N2-O2-Brine-Carbonates in Conditions from Surface to High Temperature and Pressure
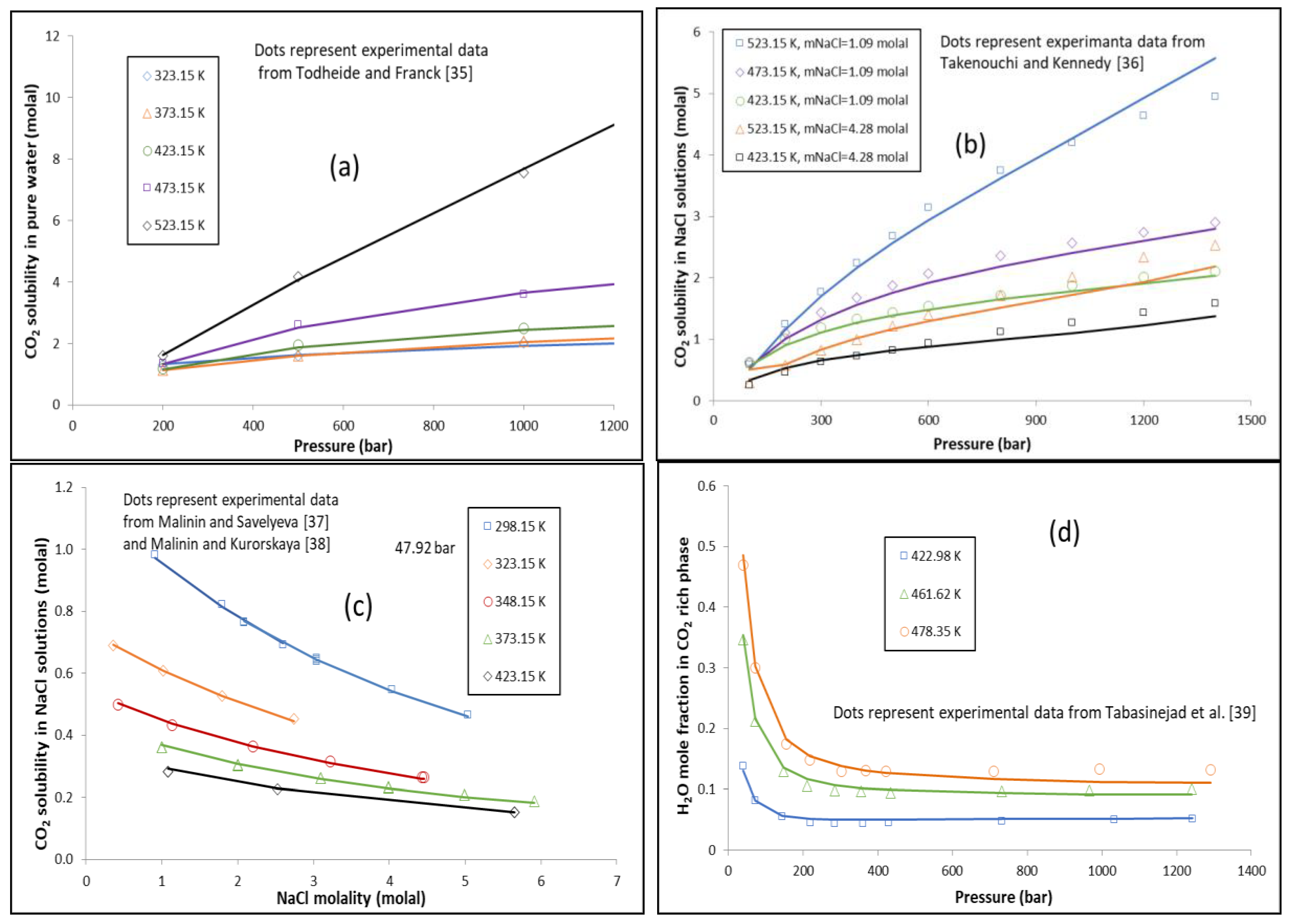
Energies | Free Full-Text | Thermodynamic Modeling of CO2-N2-O2-Brine-Carbonates in Conditions from Surface to High Temperature and Pressure
![The solubility (S) of dolomite [CaMg(CO 3 ) 2 ] as a function of pH... | Download Scientific Diagram The solubility (S) of dolomite [CaMg(CO 3 ) 2 ] as a function of pH... | Download Scientific Diagram](https://www.researchgate.net/profile/James-Altland/publication/309875485/figure/fig1/AS:537898228826112@1505256339484/The-solubility-S-of-dolomite-CaMgCO-3-2-as-a-function-of-pH-LlogH-D_Q320.jpg)
The solubility (S) of dolomite [CaMg(CO 3 ) 2 ] as a function of pH... | Download Scientific Diagram

Calcite, dolomite and magnesite dissolution kinetics in aqueous solutions at acid to circumneutral pH, 25 to 150 °C and 1 to 55 atm pCO2: New constraints on CO2 sequestration in sedimentary basins - ScienceDirect

Low-Temperature Synthesis of Disordered Dolomite and High-Magnesium Calcite in Ethanol–Water Solutions: The Solvation Effect and Implications | ACS Omega

Calcite solubility in aqueous solution with CO2 in equilibrium obtained... | Download Scientific Diagram
![The solubility (S) of dolomite [CaMg(CO 3 ) 2 ] as a function of pH... | Download Scientific Diagram The solubility (S) of dolomite [CaMg(CO 3 ) 2 ] as a function of pH... | Download Scientific Diagram](https://www.researchgate.net/publication/309875485/figure/fig1/AS:537898228826112@1505256339484/The-solubility-S-of-dolomite-CaMgCO-3-2-as-a-function-of-pH-LlogH-D.png)
The solubility (S) of dolomite [CaMg(CO 3 ) 2 ] as a function of pH... | Download Scientific Diagram

The dissolution rate of dolomite as a function of aqueous calcium (open... | Download Scientific Diagram

Figure SI-1.3 Solubility of the magnesian calcites as a function of the... | Download Scientific Diagram

Solubility diagram for the system calcite/Ca-rich dolomite at T = 10°C... | Download Scientific Diagram

SE - Precipitation of dolomite from seawater on a Carnian coastal plain ( Dolomites, northern Italy): evidence from carbonate petrography and Sr isotopes
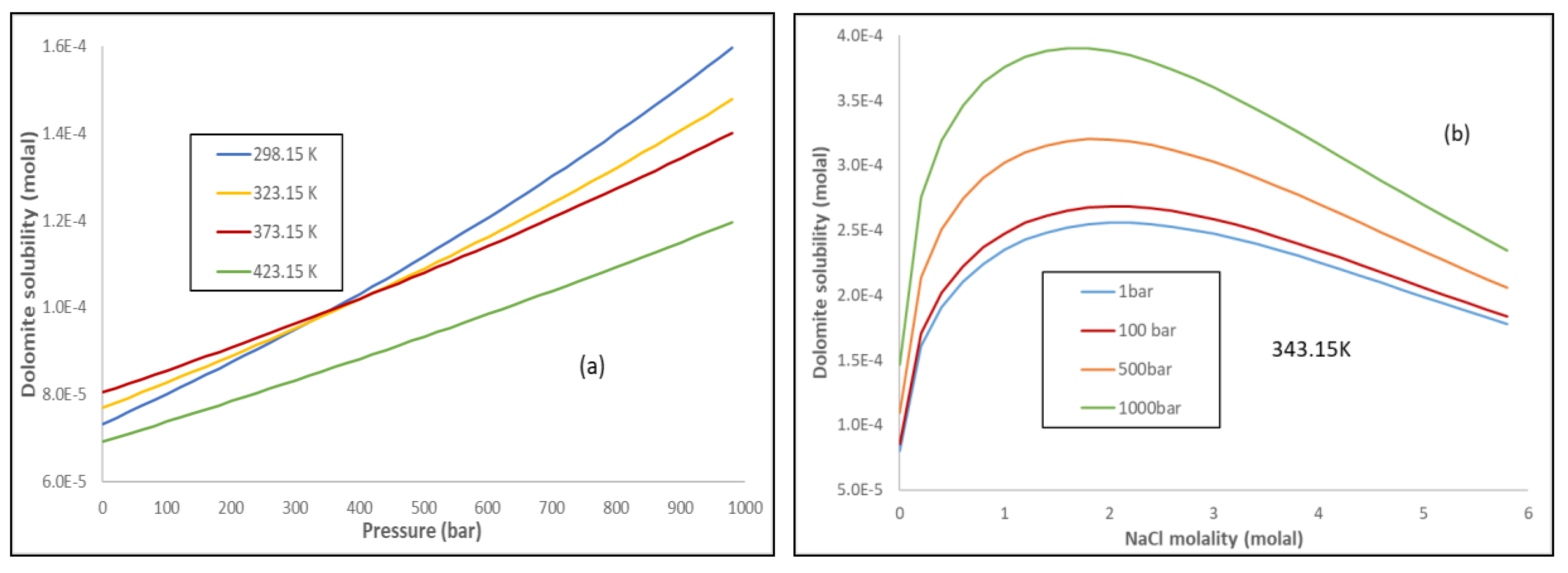
Energies | Free Full-Text | Thermodynamic Modeling of CO2-N2-O2-Brine-Carbonates in Conditions from Surface to High Temperature and Pressure

Dissolution kinetics of calcite, dolomite and magnesite at 25 °C and 0 to 50 atm pCO2 - ScienceDirect